We are just finishing up a chemistry unit in science called Very Reactive. We were split into groups of 3 and assigned a kind of chemical reaction to research. My group was given decomposition reactions. These reactions happen when a single compound is broken up into two or more new compounds or elements. Throughout this unit we used a digital textbook to take notes and learn about the concepts. Through the digital textbook, I learned all about the different kinda of reactions that form.
While researching decomposition reactions, our group found the perfect one to use for our experiment. We decided to decompose water through electrolysis. The school happened to have the right machine for it too, which made our lives easier. This experiment involved taking water and adding acid (to speed up the reaction) and then running electric currents through the water. We did this by attaching a battery to a machine like this:
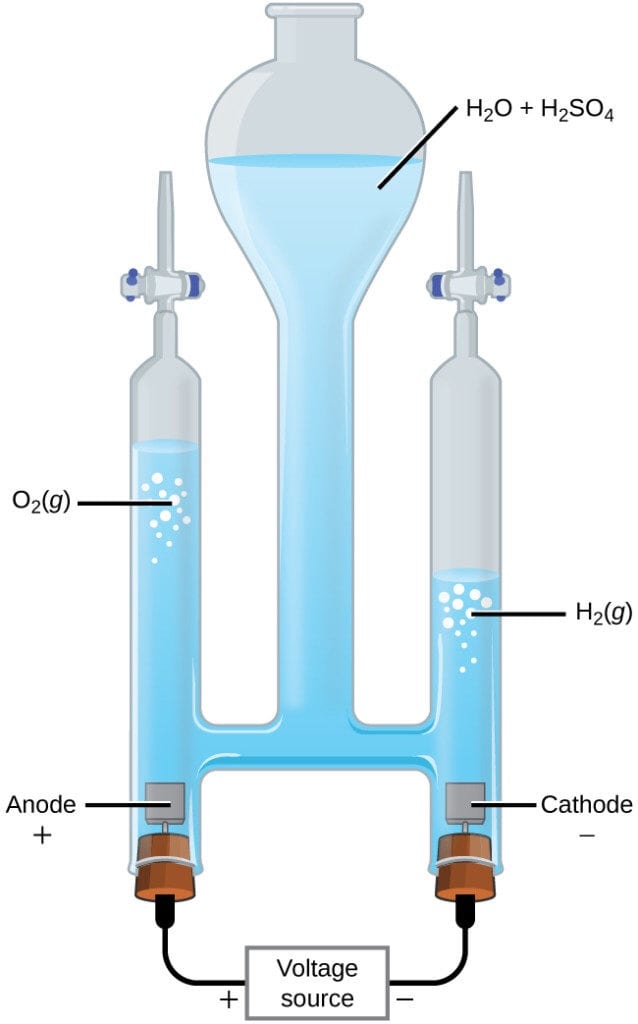
Then, we turned on the power source and let the machine sit for a few minutes. What happened in this process was the water decomposed into it’s hydrogen and oxygen molecules, which rose to the two separate sides of the machine. There was twice as much hydrogen for every oxygen in the machine, since water’s molecular structure is H2O. We then emptied the hydrogen and oxygen (one at a time) into a test tube. We burned the hydrogen off by lighting it with a lighter, creating a popping sound. We put the oxygen in a different tube and then took a toothpick and lit it on fire. We blew it out so it was just an ember, then put it into the test tube and it relit due to the amount of oxygen.
We also learned about other reactions in this project. Those reactions are synthesis, single replacement, double replacement, acid base neutralization, and combustion. You can learn all about those in my project end mind map right here!

Here are the competencies that I used for this unit:

