This latest scimatics project that we completed was all about Chemistry but chemistry coding in particular, but what is “Chemistry Coding”?. The big idea for this project was “the behaviour of matter can be explained by the kinetic molecular theory and the atomic theory”. Our driving question was “How is the motion of atoms and molecules related to temperature”. Our final project was to make a interactive scratch applet that showed atoms, molecules and the three different states of matter. You can find my scratch game here and my project mind map below.
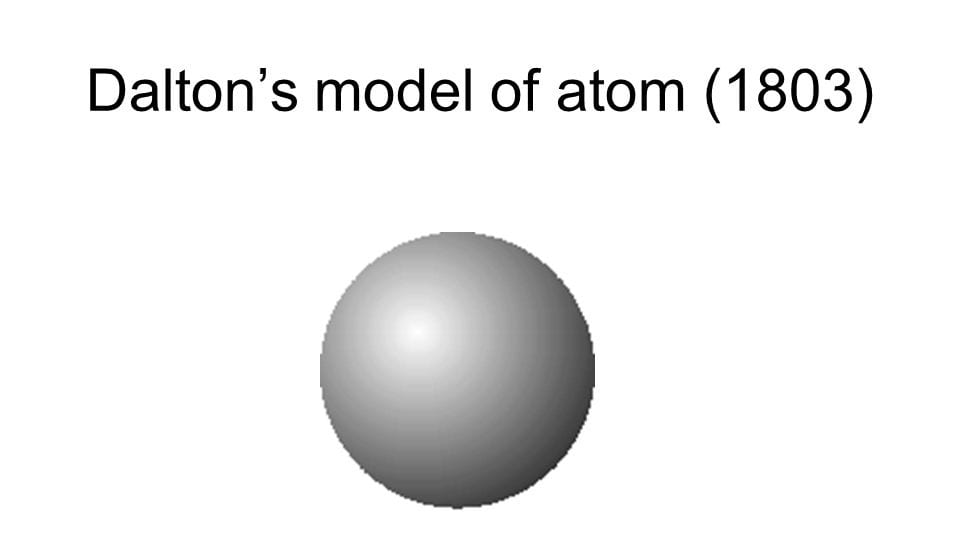
My understanding of the Kinetic molecular theory and the atomic theory starts at the first theories and models of atoms. The first one was the solid sphere model by John dalton in 1803 pictured below. His theory stated that atoms are indivisible which turned out to be incorrect. He also stated that atoms of a given element are identical and compounds are combinations of different types of atoms.
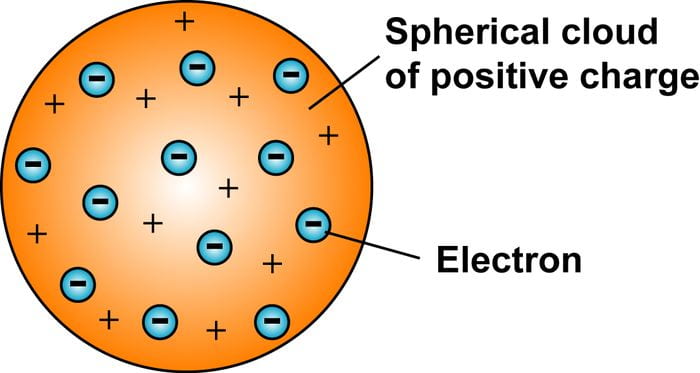
The next picture below was J.J Thomson’s plum pudding model in 1904. This model was a much better representation of an atom compared to John Dalton’s model. J.J’s model showed that an atom was composed of electrons scattered throughout a spherical cloud of positive charge. He did recognize that electrons were components of atoms but the lack of a nucleus in his model didn’t explain later experimental observations.
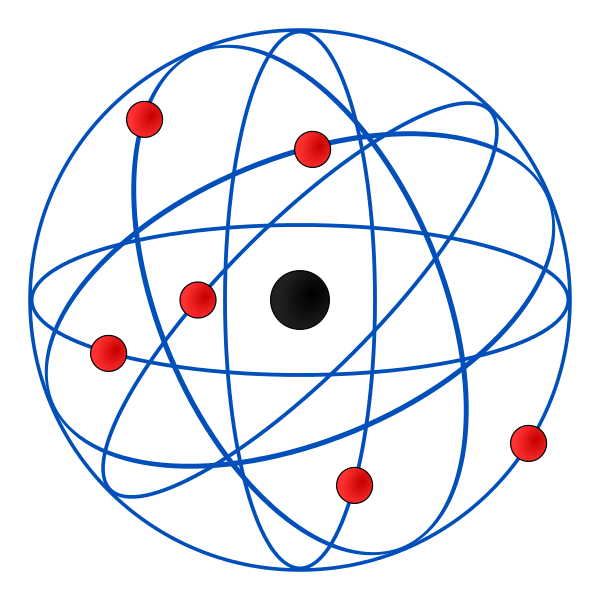
The third model is Ernest Rutherford’s Nuclear Model in 1911. This model shows electrons orbiting around the nucleus. Rutherford’s famous experiment was when he fired positively charged alpha particles at a thin sheet of gold foil. A lot of the particles passed through with little deflection but some deflected at big angles. He concluded that this was only possible if the atom was mostly empty space with the positive charge in the centre at the nucleus. One issue was the he did not explain why electrons remain in orbit around the nucleus.
Just two years later in 1913 was Niels Bohr’s Planetary Model. Bohr modified Rutherford’s model of the atoms stating that electrons moved around the nucleus in orbits of fixed sizes and energies. The problem with his theory was that moving electrons should not emit energy and collapse into the nucleus.
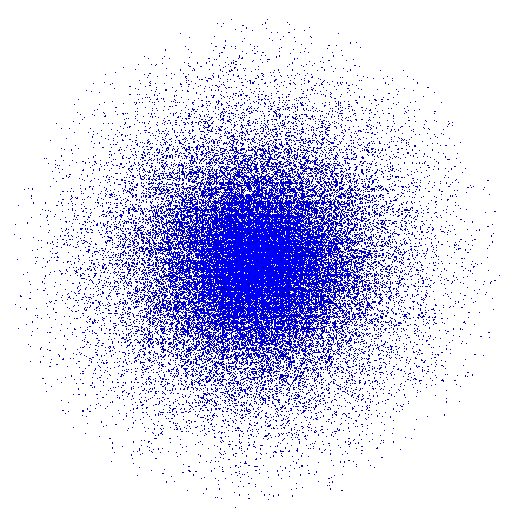
The fifth and final Model was the Erwin Schrödinger Quantum Model in 1926 pictured below. Schrödinger stated that electrons do not move around in set paths around the nucleus but in waves. Since it is impossible to know the exact location of the electrons we instead have what Schrödinger called “clouds of probability” in which we are more likely to find and electron. This model is still widely accepted as the most accurate model of the atom.
Something that I learned that I thought was very interesting was that you can actually reach absolute zero. Absolute zero is -273.15˚ Celsius (-469.67 Fahrenheit) or 0 Kelvin. The reason is that it would take and infinite amount of time and effort. Competencies: In the questioning and predicting competency I used my class time efficiently without distractions. In the Scientific communication competency I used several different atoms in my coded applet and and different states of matter but at first my different states of matter were not clear but I revised and made them very clear. The third competency was the reasoning and analyzing competency for this my scratch coded matter simulator was created with logical conditions and functional user controls. Overall I think that this project was pretty cool but I think that I still could learn more about the concept of the Kinetic Molecular Theory and the Atomic Theory.


Leave a Reply