During this term in science we learned all about the periodic table and the elements within it.
Just like in the beginning of every term in science, we created a mind map of everything we now about the topic before we have been taught anything. And I obviously didn’t know much.
Then we began to take notes on:
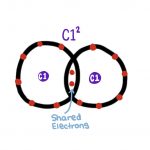
Covalent Bonds: the sharing of electrons between pairs or groups of
atoms
Ionic Bonds: The bond between two atoms with different charges, one atom gives up one or more electrons to another atom
Bohr Models: a model of the atom created by Ernest Rutherford, to show that the atoms electrons travel in a circular orbit, similar to the solar system
show that the atoms electrons travel in a circular orbit, similar to the solar system
 Electrons: the particle of negative energy found in the atom
Electrons: the particle of negative energy found in the atom
Protons: a particle in the nucleus of the atom with a positive charge
Neutrons: a particle with the same mass as a proton but without an electric charge, found in the nucleus of the atom
These are just the basics.
After the notes and lectures this is what my mind map looked like.
Next, to show our learning, we paired up and created a video about atoms as if they were characters. My partner was Adlih and ours was about about a little atom who had to walk to school by himself and gets bullied by another atom who is bigger because of his full valence shell, then he meets a friend and they share an ionic bond.
My knowledge of chemistry really improved during this term and I’m looking forward to continuing this topic next term