Adlih Britton: 24825358 Dec 15, 2023
Welcome to Just a Touch of Catalyst! I am excited to embark on this weeks article where we will be discussing some important inorganic chemistry reactions. Excitingly, some of this week’s work was published by Dr. Laurel Schafer, a professor at UBC1! I highly recommend you take a look at some of her impactful work.
As always get comfortable, make yourself a tea and we’ll get into the nitty gritty of two very important inorganic reaction mechanisms.
When baking I often find myself shocked with the time certain recipes take. I can’t be the only one who sits down to make something and takes one look at the recipes and is gobsmacked! 3 hours? I don’t have that kind of time! Especially if I am not confident that the recipe will produce anything that tastes very good! What if I mess up? What if the food I make just doesn’t taste very good? Well then what a waste of time and ingredients. It would be nice to have a recipe that could guarantee a delicious food item, in a short amount of time. This conundrum sets the stage for what our chemistry will be attempting to combat today.
As always, our list of terms. Make sure to revisit these if the metaphors become confusing!
| Chemistry Term | What is it? (Chemistry) | Cooking Equivalent |
| Catalysis | A mechanism for making a molecule, that uses something and regenerates that thing7 | Using an ingredient to accelerate the process, but not to use in the food |
| Hydroamination | A catalytic reaction that can react a nitrogen with a double bond or triple bond6 | A recipe for a more complex meal, which reacts a carbon with a nitrogen |
| Hydroaminoalkylation | A catalytic reaction that can react the carbon next to a nitrogen with a double bond or triple bond8 | A recipe for a more complex meal, that involves a reaction beside the nitrogen |
Now that we’ve gotten the terms out of the way, lets get started with these wonderful reactions!
You may be wondering why I ranted to you about long cooking times and yucky tasting meals above. Aside from this being a regular occurrence, these issues are the premise of using the reactions mentioned above. In chemistry, alike cooking, some reactions can be challenging and as such can invoke long waiting periods. These long periods can be upsetting to a researcher if they are trying to make an important molecule. Just like how you might be upset if you have pulled up a meringue recipe for a dinner party and realize they are going to take you 3 hours to make, you might not be able to finish them in time! Without adequate preparation this meringue cook may miss the dinner party which would mean the efforts were (in essence) wasted. Like cooks heading to a dinner party, chemists would like reactions to occur in a timely manor such that they can create important molecules efficiently. The reactions we will be discussing today provide efficient routes to many important molecules, because they provide creative ways to react a nitrogen. Roughly 40% of pharmaceutical drugs and pharmaceutical drug candidates have carbon-nitrogen bonds2 meaning they are extremely important in pharmaceutical drug design and synthesis. As a reader you may be wondering why should I care about how many pharmaceutical drugs have nitrogens? Well to that I might say that pharmaceutical drugs are extremely important in todays society and most people will interact with them tons throughout their lifetime! Take for example a bacterial infection, prescribed antibiotics such as Penicillin3 or Cefalexin4 will ensure a speedy recovery. Both of these drugs have carbon-nitrogen bonds that can be challenging to make, requiring some creative syntheses which is where the reactions we will be discussing come into play!
Pictured below are both the chemical structures for Penicillin or Cefalexin. If these structures are confusing or too complicated, pay little attention to them! They are merely here to provide the reader with evidence of the carbon nitrogen bonds found in pharmaceutical drugs. 
Without pharmaceutical drugs, a small infection may lead to serious longterm health impacts5, which goes to highlight the important role they play in our lives. People require pharmaceutical drugs for a myriad of reasons, though without adequate avenues to make these pharmaceutical drugs we wouldn’t be able to rely on them. Therefore it becomes extremely important to develop synthesis to make these important molecules as efficiently as possible which is where hydroamination and hydroaminoalkylation, the reactions we are discussing today, come in. Though pharmaceutical drug synthesis is an expansive topic I believe it is essential to understand why these reactions are so important to us, and you can’t get that without getting the role they play in pharmaceutical drug synthesis! For the duration of this article we will leave pharmaceutical applications behind and get into the nitty gritty of the reactions mechanisms!
Hydroamination and hydroaminoalkylation provide efficient reaction pathways to important molecules! Switching back to our baking analogy, say we wanted to make meringues but the recipes were going to take too long, and we still had that dinner party to attend. We’d be looking for an efficient, delicious, recipe for meringues, this is how we can think of the aforementioned reactions. Lets begin by discussing the hydroamination reaction!
 Hydroamination is just a fancy word for a reaction that adds a carbon, previously in a double or triple bond, to a nitrogen6. This reaction falls into the category of organometallic reactions, which means it involves carbons as well as metals6. The metals that contribute to this reaction contribute in the form of catalysts, which means they increase the reaction rate, but don’t end up in the product7. Funny concept, isn’t it! If you’re trying to conceptualize a catalyst, try thinking about it as a spoon in a cooking sense. If your attempting to mix ingredients in a bowl it would be faster to use a spoon then to just stare at the bowl and wait for gravity and diffusion to do the mixing for you! In chemistry when reactions are slow or will not proceed often a catalyst is used to expedite the process and guide the molecules to the product. Though importantly, the catalyst doesn’t end up in the product, its is regenerated, similar to a spoon in cooking, it is used and then can be reused again once washed! In the case of the hydroamination reaction, the metals increase the reaction rate for the process of adding the carbon to the nitrogen.
Hydroamination is just a fancy word for a reaction that adds a carbon, previously in a double or triple bond, to a nitrogen6. This reaction falls into the category of organometallic reactions, which means it involves carbons as well as metals6. The metals that contribute to this reaction contribute in the form of catalysts, which means they increase the reaction rate, but don’t end up in the product7. Funny concept, isn’t it! If you’re trying to conceptualize a catalyst, try thinking about it as a spoon in a cooking sense. If your attempting to mix ingredients in a bowl it would be faster to use a spoon then to just stare at the bowl and wait for gravity and diffusion to do the mixing for you! In chemistry when reactions are slow or will not proceed often a catalyst is used to expedite the process and guide the molecules to the product. Though importantly, the catalyst doesn’t end up in the product, its is regenerated, similar to a spoon in cooking, it is used and then can be reused again once washed! In the case of the hydroamination reaction, the metals increase the reaction rate for the process of adding the carbon to the nitrogen.
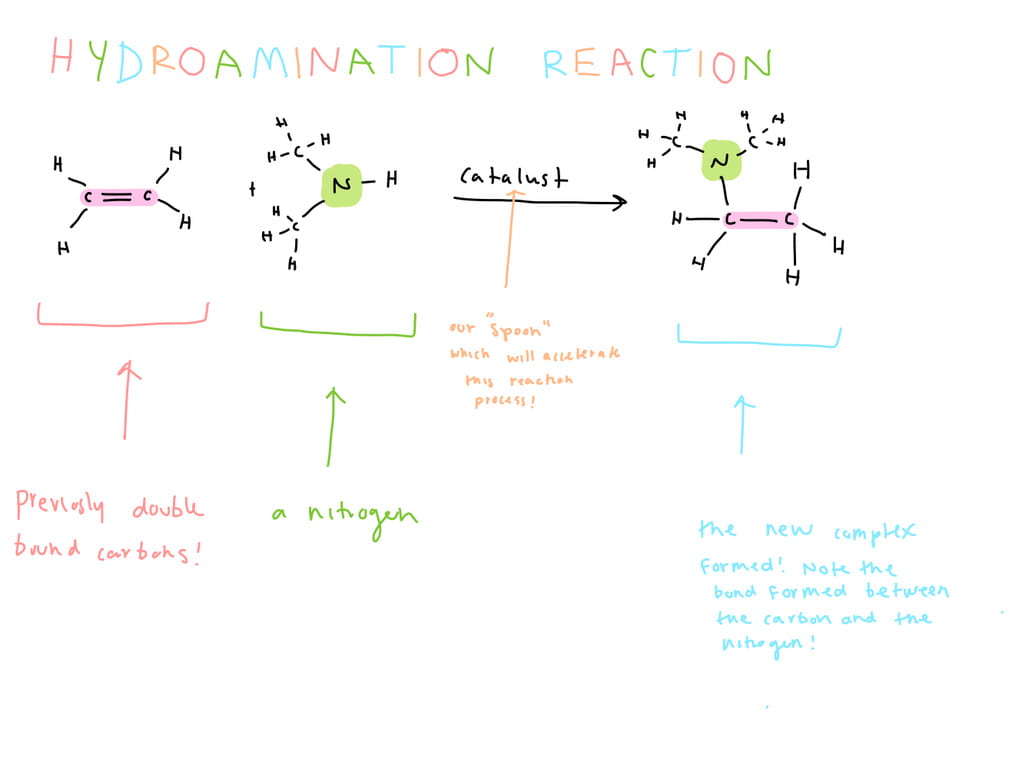
An example of the hydroamination reaction is provided below. Let’s break it down!
On the far left in pink, you can see the double bonded carbons. This is what we are going to add to the nitrogen. Beside the carbon double bond we can see the nitrogen that we are going to bind to the carbon from the double bond. Over the arrow we can see our catalyst, or our “spoon.” Lastly we can see the product where we have nitrogen added to the carbon. The hydroamination reaction allows for the reaction of a nitrogen at a relatively un-reactive carbon6. A reaction accomplishing this is highly sought after because many important molecules require reacting nitrogens at relatively complex and un-reactive carbon centres, like the pharmaceutical drugs we discussed earlier!
If we return to our cooking analogy briefly, lets set the hydroamination reaction aside as our meringue recipe. We’ve found a terrific recipe to making meringues, a complex dessert item, easier! Though what if we wanted to add a topping to our meringues, or cover them in whipped cream. How might we consider this added challenge, and what recipe should we use?
Breaking down our new problem into bite sized pieces, we need to find a new recipe that will add some flair and flavour to our original meringues, while also ensuring that the flavour profiles match. We might be inclined to find a similar recipe, that will alter things just so, that our final product is different.
This (though confusing) is my attempt at an analogy for the the hydroaminoalkylation reaction, which is a tad trickier to describe. The hydroaminoalkylation reaction differs from the hydroamination reaction because instead of reacting off the nitrogen, it reacts off the carbon right beside the nitrogen7, this gives the product some new flair! The hydroaminoalkylation reaction then opens the doors further to challenging syntheses that invoke carbon-nitrogen containing compounds7.
The hydroaminoalkylation reaction, pictured below, reacts at the carbon beside the nitrogen, instead of the nitrogen itself, so let’s go through it!
As you can see this reaction adds the carbon beside the nitrogen which differs from hydroamination where the carbon was added to the nitrogen! Within the hydroaminoalkylation reaction a catalyst is used to increase the reaction rate. Between the hyrdoamination reaction and the hydroaminoalkylation reaction the catalysts will differ, though overarching aspects of the catalyst will remain constant. Both reactions will have catalysts made out of metals that have been found to have high activity, meaning they are active in the reaction and wield their electrons in a way that complements the substrate7! In addition, both hydroamination and hydroaminoalkylation make use of molecules on the catalyst. These molecules job is to help direct movement and ensure the product formation. Between these two reactions the differences in the catalyst appear when looking at the metal chosen as well as the molecules chosen to compliment it. Many research groups have found unique catalysts that preform specific hydroamination and hydroaminoalkylation reactions1,8 and continued research on catalytic design is a very important aspect of inorganic chemistry!
If we return to our baking analogy we could say that, with the hydroaminoalkylation reaction, we have found a recipe that will add that extra flair to the meringues. The recipe provides the requred differences from our standard recipe efficiently! This means we have now come up with a rather complex recipe for very important meringues with a topping for our dinner party.
With both of these reactions in our back pockets the process of making these complex substances can be easier! If we transition back to the chemistry we have discussed, looking at the importance of the reactions we can see that both hydroamination and hydroaminoalkylation provide reaction pathways that focus on reacting nitrogens (whether that be on or beside!). Revisiting what we discussed at the beginning, nitrogen containing molecules are extremely important in pharmaceutical drug design, and so these reactions can provide efficient reaction pathways to important molecules. Therefore the reactions we have discussed today are assets for drug discovery!
Now that we’ve gotten through all that chemistry you deserve some meringues! You might want to try out this recipe! (Nudge-nudge, look at the time required!)
Citations
- Hao, H.; Schafer, L. L. Metal–Ligand Cooperativity in Titanium-Catalyzed Anti-Markovnikov Hydroamination. ACS Catalysis 2020, 10 (13), 7100–7111. https://doi.org/10.1021/acscatal.0c00491
- Research could enable assembly line synthesis of prevalent amine-containing drugs | Chemistry at Illinois. chemistry.illinois.edu. https://chemistry.illinois.edu/news/2022-04-15/research-could-enable-assembly-line-synthesis-prevalent-amine-containing-drugs.
-
Yip, D. W.; Gerriets, V. Penicillin. PubMed. https://www.ncbi.nlm.nih.gov/books/NBK554560/#:~:text=Penicillin%20is%20a%20medication%20used.
-
Australia, H. Cefalexin (Cephalexin). www.healthdirect.gov.au. https://www.healthdirect.gov.au/cephalexin#:~:text=Cefalexin%20(also%20known%20as%20cephalexin (accessed 2023-12-15).
-
What Would Our World Be Like Without Medicines? | Twist Bioscience. www.twistbioscience.com. https://www.twistbioscience.com/blog/perspectives/what-would-our-world-be-without-medicines.
-
Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Catalysis/Catalyst_Examples/Hydroamination#:~:text=Hydroamination%20is%20a%20reaction%20that (accessed 2023-11-05).
-
Catalysts. Chemistry LibreTexts. https://chem.libretexts.org/Bookshelves/Inorganic_Chemistry/Supplemental_Modules_and_Websites_(Inorganic_Chemistry)/Catalysis/Catalysts.
-
DiPucchio, R. C.; Rosca, S.-C.; Schafer, L. L. Hydroaminoalkylation for the Catalytic Addition of Amines to Alkenes or Alkynes: Diverse Mechanisms Enable Diverse Substrate Scope. Journal of the American Chemical Society 2022, 144 (26), 11459–11481. https://doi.org/10.1021/jacs.1c10397.



Published by