Unit 2 introduction Notes package: Chem 12 EQUIL intro package
Worksheets to understanding the two driving forces of a spontaneous reaction at equilibrium:
Enthalpy vs Entropy a Second Look
Enthalpy vs Entropy a Second Look KEY
Looking for EVEN MORE extra practice in understanding the opposing forces of minimum enthalpy and maximum entropy in a Dynamic Equilibrium? equil-entropy-enthalpy-worksheet and equil-entropy-enthalpy-worksheet-answer
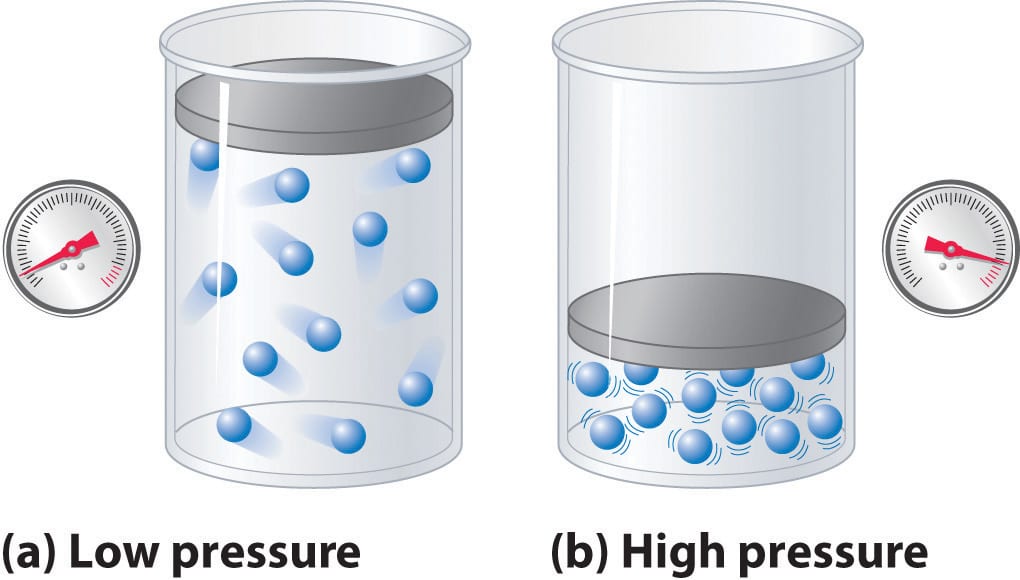
Take a look at this simple analogy site that helps you to understand ENTROPY: https://aatishb.github.io/entropy/
For the Video BELOW, Stop watching the following video at 4:30. Up until then, it demonstrates nicely how to predict maximum entropy (or the direction of increasing entropy). After 4:30, it switches to “AP” content.
THINK OF EQUILIBRIUM LIKE THIS: https://dribbble.com/shots/6067584-Teeter-Totter
An introduction to the stoichiometry of reversible reactions (“IRE” or “ICE” analysis) IRE grids intro handout and IRE grids intro answer key.
We will take another look at IRE or ICE grids and relate them to the ever important value: K equilibrium. This is an enduring concept for the rest of the course! Derivation of K notes after IRE analysis
You MUST feel 100% confident about this entire package: K special notes and prac calcs and ANSWER KEY K calcs and special notes Extra K practice: MoreKQns2.2 and MoreKQnsKEY and this package.
For those students looking for more: Ch12 Extra Worksheet K IRE grids and answer key: Extra K IRE Worksheet Package KEY
for those students looking for more: Ch12 Extra Worksheet K IRE grids and answer key: Extra K IRE Worksheet Package KEY
Here are a couple of videos that talk about what DYNAMIC EQUILIBRIUM IS:
Spectrophotometer / Equilibrium lab experiment. Often a full formal lab report is assigned for this lab.
Chem12 FeSCN LAB Lab2.1Table CascadeDilution Chem 12 FeSCN Report CRITERIA
How does a specrophotometer work?
What our Spectrophotometer Lab looks like:
Read and understand this two sided handout: Le Chatelier Principle NOTES. This is a GAME CHANGER for Unit 2!!!!!
Mrs. Toombs will do a demo to show you how temp can SHIFT an equilibrium. This handout goes with the demo: NO2 to N2O4 FULL notes. Be sure that you can answer the 5 analysis questions.
A video showing what the NO2 to N2O4 demo looked like:
Starting to talk about STRESSES and SHIFTS on Equilbrium reactions (Le Chatelier’s Principle). (These notes all occur on the front whiteboard, as you need to understand each scenario as it is played out).
Print out some PRETTY full colour notes that guide you through LeChatelier examples: LeChatgraphnotes
Le Chatelier’s Principle explained: (this video talks about STRESS)
Le Chatelier’s Principle explained: (this video talks about STRESS when energy is changed)
CHEM12STRESSWorksheet2.3 and Worksheet 2.3 KEY
LeChâtelier’s Principle STRESSFUL examples and LeChâtelier’s Principle STRESSFUL examples ANS KEY
And one more worksheet: Graph O Rama and Graph O Rama ANS
LeChatelier and K calcs 2020 K calcs / ICE GRIDS that include STRESSES
ANSWER KEY to K calcs that include STRESSES on the ICE grids. NOTE! You will not see all of these questions on the packet that you receive in class. Ignore any answers that are included here, that are not in your photocopied version of this worksheet package: Le Chatelier and K calcs updated ANS KEY
EXTENSION: Read pages 324 to 329. For those who don’t have your textbook handy, here is the reading from the final page, p. 329: HABER AND MORALITY OF SCIENCE. You might also want to do some background research (on Wikipedia, or other) on Fritz Haber, Albert Einstein, and Alfred Nobel. Pay close attention to the Scientific work they did and how it impacted chemical warfare.
youtube.com watch?v=ztzKHU2oaF8
Click on the following image to see this incredible resource!
Reversible Reactions, Equilibrium, and Le Chatelier’s Principle
REVIEW PACKAGE TO PRINT OUT FOR YOURSELF:
(Before you hit print: this is a 5 page package on 8.5X14 paper!) Here is an excellent (thank you very much  ) review package to practice for this test: ch12 unit 2 review pkg and Ch 12 U2 Review pkg ANS KEY.
) review package to practice for this test: ch12 unit 2 review pkg and Ch 12 U2 Review pkg ANS KEY.
NOTE: Although this is primarily a multiple choice review, use it to assess your understanding as you work through each multiple choice question. (Explain your answer to yourself out loud, explain the WRONG answers to yourself out loud).
Don’t overlook the short answer questions on the final page as a thorough concept review.